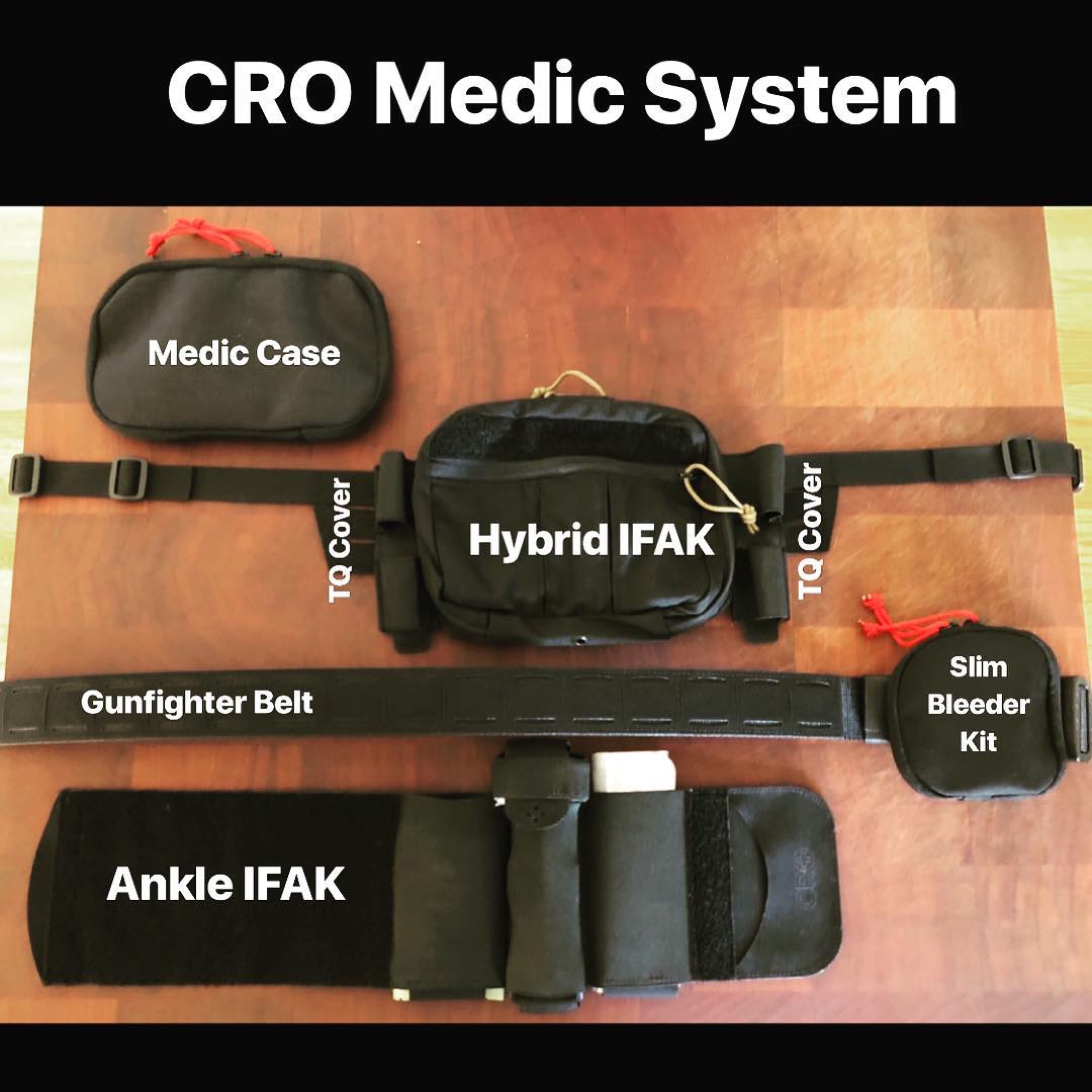
CRO Medical was started to identify, design and develop medical devices for combat medics. In January 2017, the Committee on Tactical Combat Casualty Care (CoTCCC) introduced pelvic binding into the protocols due to the high instance of pelvic fractures from common battlefield injuries. 26% of service members that died in OEF/OIF had a pelvic fracture. If you follow the principles of good medicine, carrying a dedicated pelvic binder is an absolute necessity. With this kind of data to support routine pre-hospital pelvic binding, it goes as far as negligence if you don’t carry a binder.

The CRO Pelvic Binder, now FDA approved for commercial sale, went through at least eight versions before arriving at the production model. This design is based off a 5” wrap with a BOA dial for mechanical advantage. We decided to go with BOA due the extremely high tolerances on their hardware and proven track record of reliability. The binder can be placed by a single operator. Binding occurs over the greater trochanters and requires 33 lbs of force at a minimum to “close the book” on an open book pelvic ring fracture, but studies show that over-tightening with the BOA dial is not possible, so there is no risk of causing harm to the patient.

Recently, a third pary medical research group completed the first part of a multi-stage pelvic binding study focused on pelvic binding at point of injury for DoD medical providers. The study shows on a cadaver model, that the CRO binder is the fastest to apply and the preferred option compared to the SAM Pelvic Sling II and the T-POD pelvic binders for special operations medics. Data will be submitted to the Journal of Special Operations Medicine when complete. The CRO Pelvic Binder will be available on GSA and other DoD contract vehicles in the coming weeks. For more information please visit cromedicalgear.com or email operator@cromedicalgear.com