ADELPHI, Md. — As the world confronts the shortage of essential medical equipment caused by the COVID-19 pandemic, the internet buzzes with efforts to build makeshift ventilators, some based on the idea of a respirator invented by U.S. Army researchers more than five decades ago.

When the coronavirus epidemic began to strain the supply of lifesaving medical equipment like ventilators, online communities of technologists banded together to help small companies and even everyday people create their own emergency medical equipment.
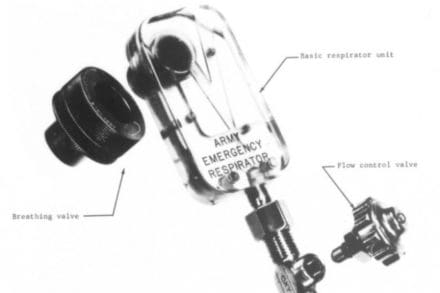
Among the many ideas and personal projects shared on internet forums, many people got excited over the design of a unique ventilator known as the Army Emergency Respirator. What caught their attention about this technology was that this particular apparatus could perform complex breathing-supporting functions without the need for any moving parts.
The Army Emergency Respirator has two configurations; a respirator with a moving bellows that takes over the intubated and sedated patients breathing, and a simple breathing assist device to help the patient breathe easier through pressure augmentation.
Army engineer Henrik H. Straub invented the device in 1964 while he worked at the Harry Diamond Laboratories, one of the seven facilities that merged to form the Army Research Laboratory in 1992.
The respirator represents one of the many important scientific milestones in the history of the U.S. Army Combat Capabilities Development Command’s Army Research Laboratory, officials said. It uses the principles of fluidics to assist or control the ventilation of the patient.
“The fluidic breathing assist device relies on the person’s labored and insufficient breathing to control the fluidic augmentation of breaths using a power-jet directed into or away from the patient’s face mask,” said Michael Scanlon, a branch chief with the lab. Scanlon began his career in the development of fluidics technology about 37 years ago when he started as a Cooperative Education student at Harry Diamond Laboratories.
Based on the theoretical foundation of fluid dynamics, fluidics allows a system to operate under a control comprised of pipes and other pneumatic or hydraulic components. Much like how electronic circuit boards use wires and electronic valves to direct the movement of electrons and govern the system’s functions, fluidic devices use small jet streams that travel along a circuit board-like structure to perform analog and digital operations. Depending on how a fluid circuit is arranged, engineers can create a variety of machines controlled entirely by the flow of liquid or gas traveling down carefully designed paths.
At the time, Harry Diamond Laboratories received a great amount of attention for pioneering the study of modern fluidics with the invention of the fluid amplifier in 1957, a device that forces a stream to follow a designated path and amplifies its power.
The apparatus named at the time the Army Emergency Respirator emerged as just one of many applications of this new breakthrough in fluidics. The device was developed by Straub and his collaborators at the Walter Reed Army Institute of Research to mainly function as an inexpensive yet reliable pressure-cycled respirator for when supplies run low.
The breathing assist device connects to a breathing mask and automatically helps the patient inhale and exhale with a feedback loop that takes advantage of the changing pressures inside of the mask.
When the air pressure inside the breathing mask is lower than outside the mask, the apparatus pulls in air from outside through a nozzle and carries oxygen into the patient’s lungs. Then, once the pressure inside the mask increases to a preset point, the apparatus automatically adjusts to help the patient to exhale, sending the air out through a different nozzle.
As a fluidic device, Straub’s invention didn’t require any moving parts. In fact, the laboratory’s prototype was only slightly larger than a pack of playing cards and consisted of a Lucite block with a system of intricate channels carved inside. However, its relatively simple design meant that it serviced as a low-cost disposable tool for routine use at hospitals and clinics.
“The elimination of moving parts in the respirator itself makes this device extremely reliable, easy to operate, and inexpensive to manufacture,” Straub stated in one of his 1965 reports.
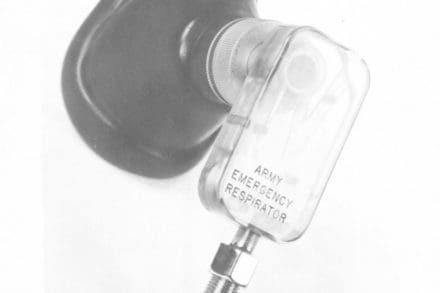
While Straub successfully tested his pressure-cycled respirator on dogs and human patients, the device remained in development as a working prototype and was never fully fielded by the U.S. Army. A similar model called the Fluidic Breathing Assistor was patented by the Bowles Fluidic Corporation in 1971, but Army research into the apparatus discontinued by the 1980s.
Despite having been confined to history for over 50 years, the renewed public attention surrounding Straub’s invention gained momentum in last few weeks as independent technologists realized its potential – and discussed it on the internet – during this time of pandemic.
One engineer has already constructed an updated version of the 1965 ventilator and shared a video of the finished product on the social media website Reddit, prompting other users to look into the design as well.
“These fluidic designs [like those featured in Straub’s pressure-cycled respirator] are so simplistic that they are suitable for mass production at negligible unit cost,” Scanlon said. “Additive manufacturing technology, such as 3-D printing with plastics, will likely enable research prototypes to be quickly and inexpensively built and tested.”
The longevity of this one invention demonstrates how foundational knowledge created within the Army laboratories can lead to an impact that extends far beyond its originally envisioned applications, and over multiple decades, officials said.
Disclaimer: The U.S. Army Combat Capabilities Development Command’s Army Research Laboratory does not approve nor recommend any medical devices and has no position on any proposed applications of the Army Emergency Respirator for any purposes.
By U.S. Army CCDC Army Research Laboratory Public Affairs