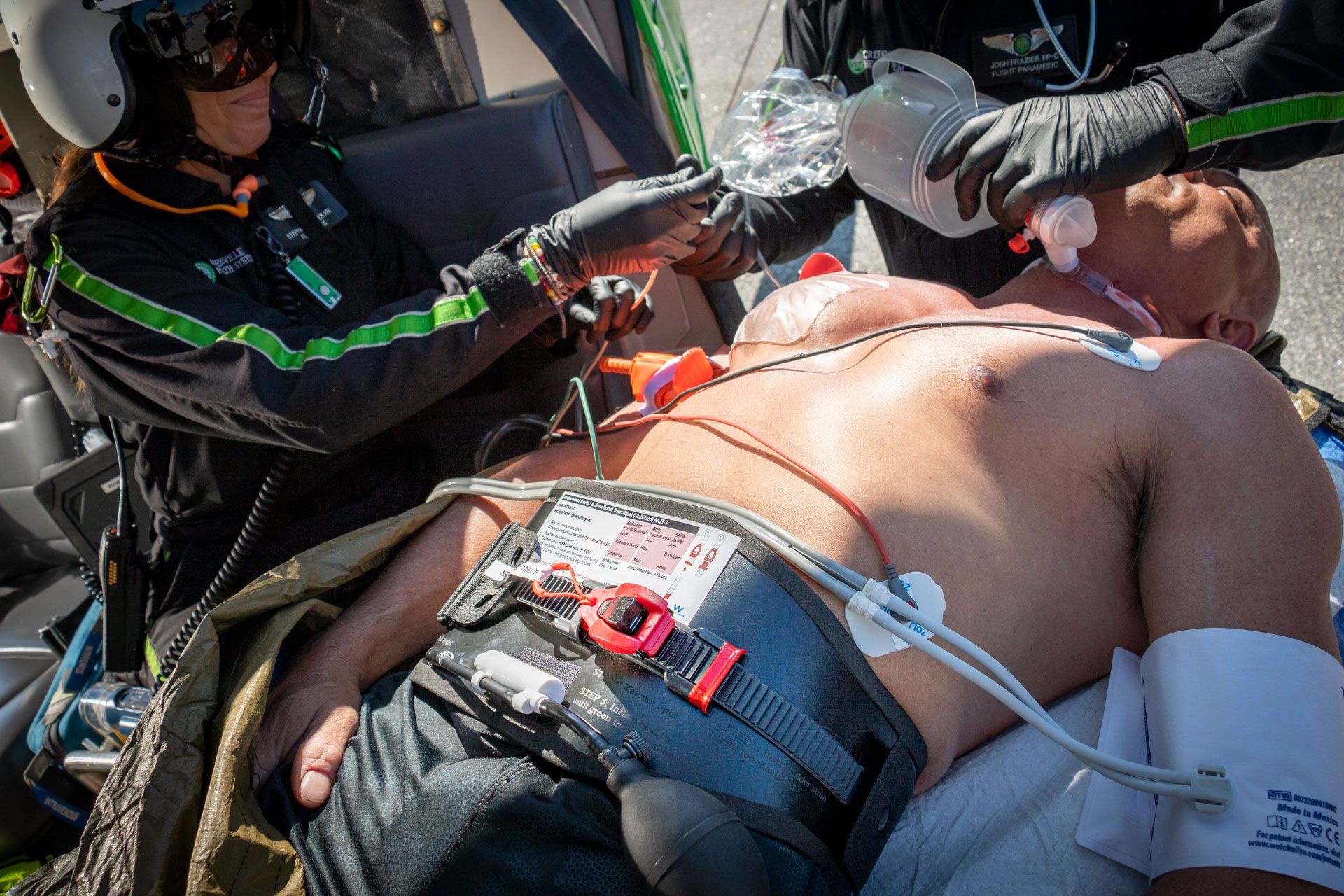
The need to be light and fast, working off your body in confined spaces applies to many medical disciplines and scopes of medicine.
It’s super interesting to see all the load carriage methods from across the services. Obviously we have a lot of crossover with SOF, both US and NATO because of our focus on austere and remote medicine in low resource areas.
Most of our tech projects and critical care resuscitation tools are primarily designed for field treatment in remote clinical medicine environments, whereas our soft goods are tailored towards operational medics who need to be light and fast, provide as many life saving treatments as possible in the shortest amount of time, while still executing mission-critical tasks.
The following describes a general framework of working off your body using our DCR Panel and Hybrid IFAK + a few tips and tricks for utilizing your pockets. The result of our expanding load carriage system is endless ways to customize and optimize your setup. Every provider is different, so what is optimal for one won’t apply to another.
At CRO, we provide a base set of tools needed to accomplish the job, across a range of different mission sets, for the operational and austere prehospital medical provider.
Hope you enjoy this post and please feel free to leave feedback in the comments.
In order of MARCH:

• TQ x1 in front of the mags, behind 40mm dangler.
Right cummerbund:


• X1 Improvised junctional (rolled SAM Splint)
• X1 Vacuum-sealed 6” ace wrap with combat gauze and compressed gauze.
• X1 Cric in CRO M4 Double Mag Insert Pouch.
Left cummerbund:


• X1 Vacuum sealed 6” ACE wrap with Combat Gauze and compressed gauze
Right ankle pocket:

• X1 vacuum sealed 4” inch ACE wrap with Combat Gauze and compressed gauze.
Left ankle pocket:

• X1 muzzle for MPC and x1 SWAT-T
Hybrid IFAK:
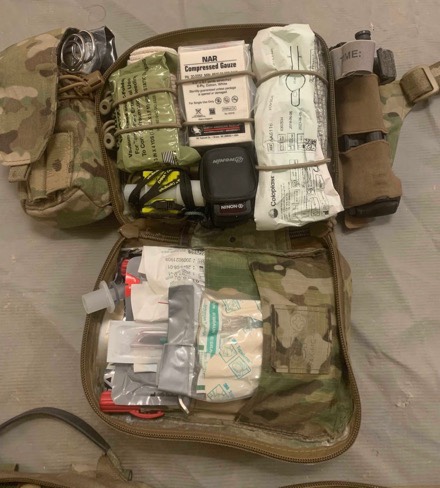
• X1 6” pressure dressing
• X1 4” ACE wrap
• X2 compressed gauze
• X1 Combat Gauze
• X1 Foley
• X1 boujie-aided cric
• X2 NPA
• X2 chest seal
• X2 10g needle D
• X1 EMMA Capnograph
• X1 pulse ox
• X1 eye shield
Mag pouch attached to left side of Hybrid IFAK:

• Hemostats and finger thor kit (vacuum sealed).
Mag pouch attached to right side of Hybrid IFAK:

• X1 FAST 1 IO
• X2 IV starter kit
Right cargo pocket:

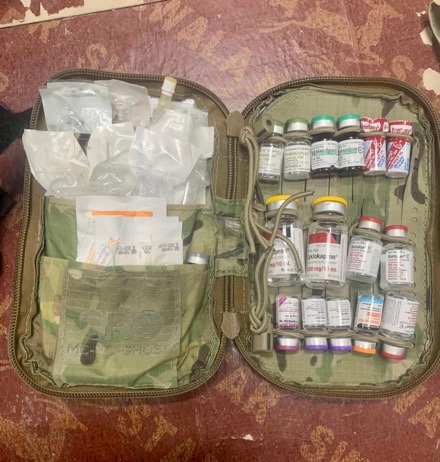
• CRO Medic Case with NARC Configuration
Left cargo pocket:
(Not Pictured)
• X2 chest seal
• X1 IOBAN
Role 1 pouch (Ferro Concepts):

• Sam Junctional so you don’t have to take off your medbag. Apply manual pressure and pull out the junctional with free hand.
DCR Panel:

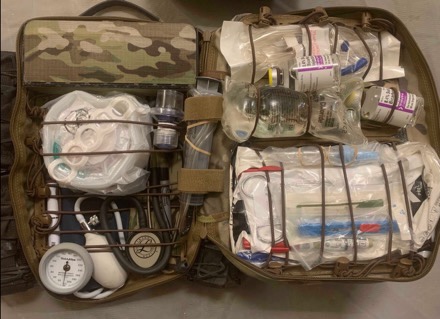

The DCR Panel is primarily used for resuscitation with blood. The purpose of working off your body and “tiering” your system up and down is to minimize the use of your bag. Ideally, you won’t need to drop your aid bag, and if you do, this means you are administering LTOWB at the POI. The aid bag is reserved for the equipment needed to resuscitate with whole blood, as well as advanced interventions, both airway and chest.
The benefit of the DCR Panel is that it is primarily clipped in, preserving shoulder mobility while being easily accessible by unclipping from the shoulders. If you are stressed for time or unable to re-clip, the hidden, low profile, “flat-straps” are available to sling the bag like a traditional aid bag.
Again, this is a general framework for optimizing your off-the-body treatment. Hopefully there are some tips that apply to your style of treatment and please let us know if you have any ways to improve.
DCR Med Bag
Hybrid IFAK
www.cromedicalgear.com