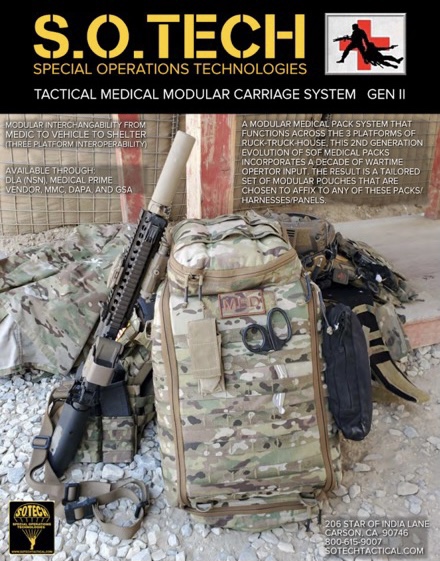
The Wolf Pack, Park Pack and First Aid Plus bring reliable protection to daily activities

PARK CITY, Utah (Aug. 10, 2021) – Uncharted Supply Co., the leader in personal preparedness, is launching three new products specifically designed to bring peace of mind to every adventure:
• Wolf Pack, a dog collar that doubles as a lightweight, durable first aid kit
• Park Pack, a modular hip pack with integrated first aid, gear repair tools and room for storage
• First Aid Plus, a waterproof and highly durable first aid kit packed with essential tools for any emergency
Building upon Uncharted Supply Co.’s revolutionary line of emergency preparedness products, this new gear will be available for purchase online via Uncharted’s website and on-shelves at official Uncharted Supply Co. retail partners across the country starting this fall. The Park Pack ($159), Wolf Pack ($89) and First Aid Plus ($124) offer a durable, yet lightweight build and give outdoor explorers (along with their canine friends) the essentials needed to safely go about daily activities.
“Since 2016, our team at Uncharted has been committed to empowering people to be the hero of their own story during emergency situations. These new additions to our line up present a more accessible and diverse way to ensure you’re prepared for the unexpected moments in daily life,” said Christian Schauf, founder and CEO of Uncharted Supply Co. “The Wolf Pack, Park Pack and First Aid Plus were built for adventurers and their dogs. Whether hiking, fishing, biking, hunting or simply exploring, these expert-designed products provide ample coverage for emergencies while enhancing (not restricting) the experience of the adventure.”

The Wolf Pack was designed specifically for furry sidekick adventurers. Created following a real-life scare that almost took the life of Uncharted’s beloved mascot, Barron, the lightweight and durable pack doubles as a dog collar and is easy for pets to wear on daily outings. It comes complete with a veterinarian-approved first aid kit to protect against cuts, bites, stings and more.

The Park Pack is a comfortable hip pack that provides protection, convenience and customization for adventures of any kind. Its welded 600D Tarpaulin shell makes it extremely durable and water-resistant, and a separate cell phone pocket sits atop the pack for easy access and protection. The pack was meticulously designed to eliminate the ‘flop’ common with many hip packs, and its EVA-molded back panel wraps away from the user’s back¾effectively eliminating uncomfortable seams. The pack comes with the Uncharted Triage Kit for first aid and gear repair, two water bottle holders and detachable water bottles positioned to stay out of the way when running or hiking. A selection of attachments for the Park Pack, soon-to-be-released by Uncharted, will transform it into the perfect kit for fishing, hunting and any other activity where lightweight transport is needed.

The First Aid Plus is an extension of Uncharted’s first aid family of products and is an essential piece of gear when heading off the beaten path. It features a waterproof, air-tight shell and the same first aid components as Uncharted’s popular First Aid Pro with the addition of a collection of tools, which include the new multi-tool, flashlight, duct tape, chem lights and more. With a Velcro tear-away Molle backer, First Aid Plus was designed to easily strap on to adventure vehicles of all kinds—taking peace of mind to the trails.
For more information on Uncharted Supply Co.’s complete line of emergency preparedness and safety gear, visit www.unchartedsupplyco.com. Visit booth #41039UL at Outdoor Retailer in Denver August 10 – 12 for a first look at the new gear.