Tactical Combat Casualty Care (TCCC) emerged in 1996 by special operations forces stemming from lessons learned during previous conflicts with large scale adoption by US and allied forces after the events of September 11, 2001. Tactical Combat Casualty Care guidelines are evidence-based and battlefield-proven to reduce deaths at the point of injury (POI). Department of Defense (DOD) and most NATO allies require TCCC training for deploying forces because it combines effective tactics and medicine to reduce preventable death. TCCC teaches first responders to treat casualties in the proper order, treating the most critical situations first. This is accomplished by using the MARCH algorithm for easy memorization for seasoned medical providers as well as immediate responders using self-aid and buddy aid. There are many variations of the MARCH algorithm that adds tasks both before and after, but the base to prevent most preventable death is MARCH.
The MARCH algorithm is laid out differently from Advanced Trauma Life Support (ATLS) which used Airway, Breathing, and Circulation (ABC’s) as the order of treatment. MARCH stands for Massive Hemorrhage, Airway, Respiration, Circulation, Hypothermia/Head injury. This order prioritizes bleeding control as the first step since morbidity and mortality linked to massive hemorrhage can happen in some cases twice as fast compared to airway and breathing complications.
What is Massive Hemorrhage?
Massive hemorrhage is the number one potentially survivable cause of death at the POI. This includes life threatening bleeding from a compressible wound and/or extremity injuries. More than 90 percent of 4,596 combat deaths after September 11, 2001 were a result of hemorrhage-associated injuries. There are many opinions and definitions of what should be considered massive hemorrhage. They include color of the blood and rate of loss but most of these are hard to qualify and quantify under the stress of the scenario combined in some cases with the operational environment and tactical context. There is always a focus of bright red bleeding vs dark red and while one is more important that the other, they both should be addressed immediately. Additionally, penetrating trauma is not selective and commonly injures both arteries and veins which present externally as a mix of bright red and dark red blood. The nature of serious bleeding leaves little time to consult the paint chart obtained from the local hardware store to compare colors and develop an appropriate treatment plan. Apply pressure! Pressure stops all bleeding.
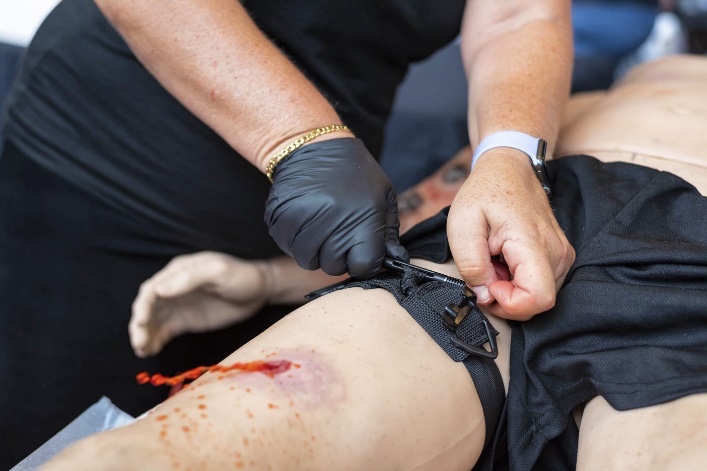
Massive Hemorrhage in the Extremities
The hasty application of a tourniquet is the recommended management for all life-threatening extremity hemorrhage during the care under fire (CUF) phase. It should be placed immediately over clothing, if necessary, proximal to the wound and high and tight. During the tactical field care phase, the deliberate application of a tourniquet is addressed when the threat has been suppressed and/or aid is being rendered behind cover to ensure proper hemorrhage control. In this phase, the tourniquet is placed against the skin, 2 to 3 inches above the wound. In either scenario the application time is written on the tourniquet at some point before the patient is evacuated or handoff is performed. Additionally, if one tourniquet is not able to control the bleeding, a second tourniquet can be placed adjacent to the first to obtain occlusion. Splinting and immobilizing the extremity after a tourniquet and pressure dressing have been applied will assist with hemostasis but should only be done after all life threats have been addressed using the MARCH algorithm and other associated treatment protocols.
External Compressible Hemorrhage
Bleeding that is not amenable to limb tourniquet use should be treated first using direct pressure in the TFC Phase until a hemostatic dressing can be applied to pack the wound. Once the bleeding is controlled, pressure should be maintained according to the manufacturer’s recommendation using manual compression, pressure dressings, or other commercially available devices.

Tools to Stop Massive Bleeding
TacMed™ Solutions offers a variety of products built to help stop the bleed including the SOF® Tourniquet, OLAES® Hemostatic Bandage, OLAES® Modular Bandage, BLAST® Bandage, ChitoGauze®, Combat Gauze, and more. Two prominent products are the SOF® Tourniquet and the OLAES® Hemostatic Bandage. The SOF® Tourniquet sets the benchmark for prehospital tourniquets with purposeful upgrades to allow for smoother and faster one-handed and two-handed applications for the most effective bleeding control. The OLAES® Hemostatic Bandage is the world’s most versatile trauma bandage by combining the globally recognized OLAES® Modular Bandage with battle tested HemCon® ChitoGauze® PRO to create the most comprehensive trauma bandage for multiple injury profiles.
Stock Your Kit to Prepare for Uncontrolled Bleeding
To stock your kit with essential tools to stop massive hemorrhaging, check out TacMed™ Solutions at tacmedsolutions.com.